A new MASCC/ISOO/ASCO Clinical Practice Guideline on Medication-Related Osteonecrosis of the Jaw has been published in the Journal of Clinical Oncology (JCO) by the American Society of Clinical Oncology. (Yarom N et al, J Clin Oncol 2019 Jul 22:JCO1901186. Epub ahead of print).
The new Guideline articulates best practices in the prevention and management of medication-related osteonecrosis of the jaw (MRONJ) in patients with cancer. A multidisciplinary Expert Panel convened jointly by MASCC, ISOO, and ASCO evaluated evidence and formulated recommendations. The literature review included studies of the prevention and management of MRONJ related to bone-modifying agents (BMAs) for oncologic indications published between January 2009 and December 2017. Results from an earlier systematic review (2003 to 2008) were also included. The resulting systematic review identified 132 publications, only 10 of which were randomized controlled trials. Recommendations underwent two rounds of consensus voting.
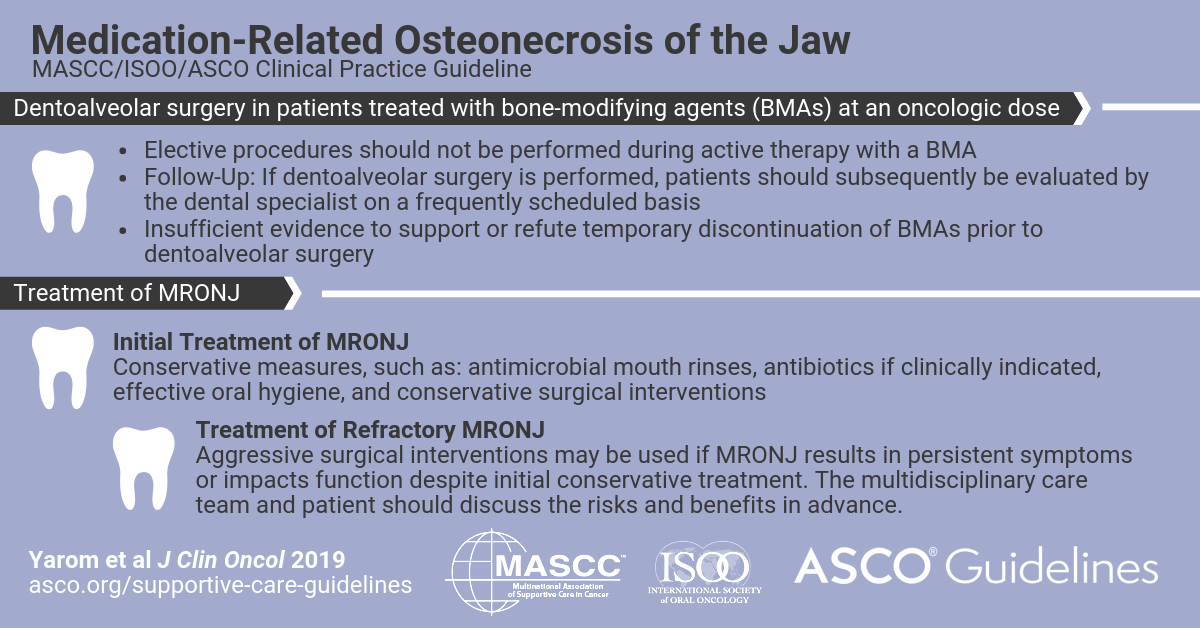
MRONJ is currently defined by (1) current or previous treatment with a BMA or angiogenic inhibitor, (2) exposed bone or bone that can be probed through an intraoral or extraoral fistula in the maxillofacial region and that has persisted for longer than 8 weeks, and (3) no history of radiation therapy to the jaws or metastatic disease to the jaws. In patients who initiate a BMA, preventive care includes comprehensive dental assessments, discussion of modifiable risk factors, and avoidance of elective dentoalveolar surgery (surgery that involves the teeth or contiguous alveolar bone) during BMA treatment. It remains uncertain whether BMAs should be discontinued before dentoalveolar surgery. Staging of MRONJ should be performed by a clinician with experience in the management of MRONJ. Conservative measures comprise the initial approach to MRONJ treatment. Ongoing collaboration among the dentist, dental specialist, and oncologist is essential to optimal patient care.